Peking University, October 24, 2024: There is a growing demand for non-invasive insights into the complex three-dimensional subcellular dynamics within living tissues on the frontier of biological research. Professor Xi Peng's group at Peking University has developed a novel imaging technique known as Multi-Confocal Image Scanning Microscopy (MC-ISM), which enhances spatial resolution, imaging depth, and minimizes phototoxicity in biological imaging.
Recent advancements have demonstrated that MC-ISM can significantly transform biological imaging techniques. By integrating confocal scanning with structural illumination super-resolution, this approach allows researchers to achieve superior imaging capabilities essential for studying living tissues.Why it matters:
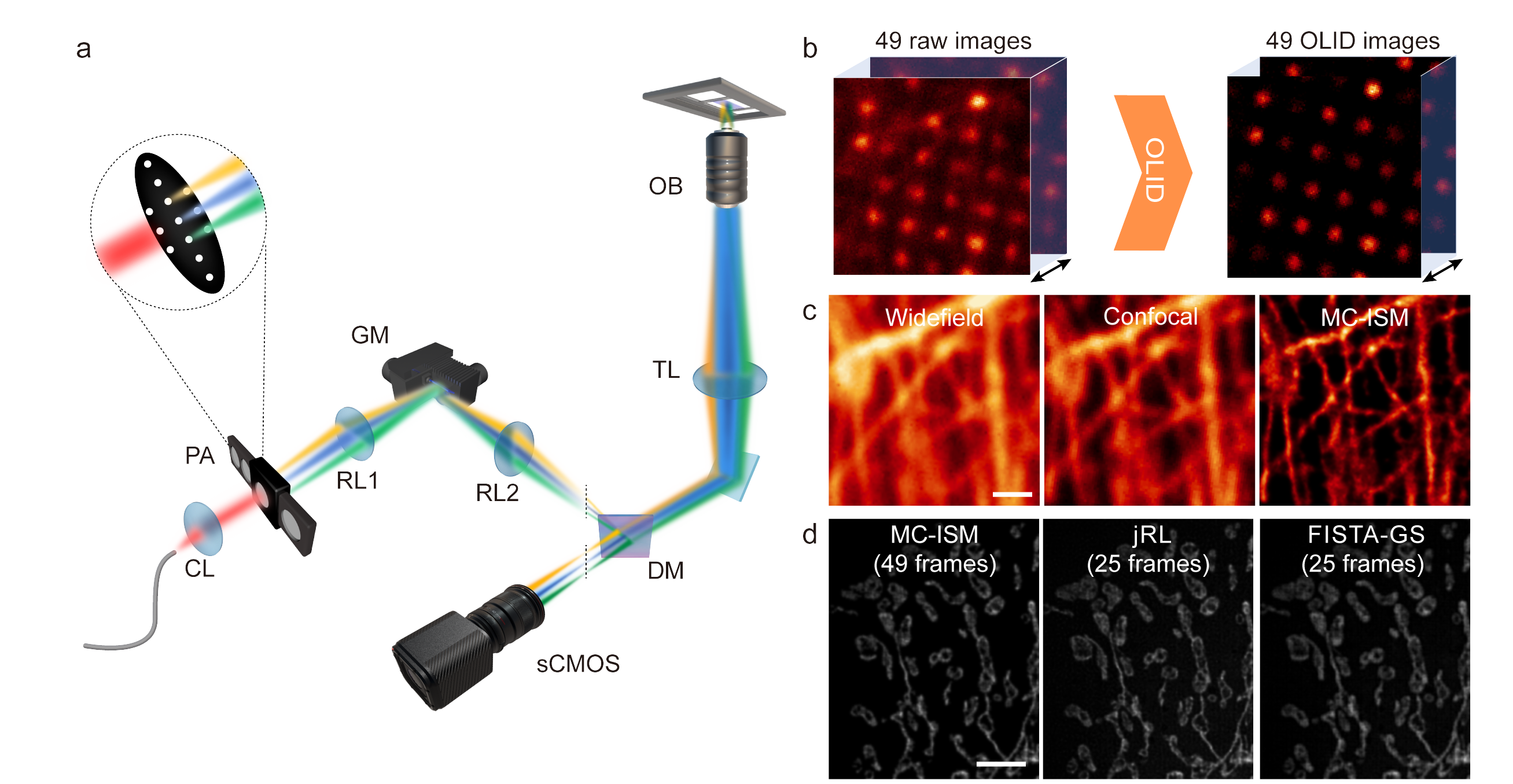
Current imaging techniques struggle to balance spatial resolution and phototoxicity while capturing rapid biological processes in living tissues. The traditional confocal laser scanning microscopy (CLSM) is limited by the trade-off between pinhole size and signal-to-noise ratio (SNR). MC-ISM addresses these challenges by integrating confocal scanning imaging with structural illumination super-resolution, allowing for enhanced three-dimensional imaging capabilities in biological specimens. This advancement enables researchers to capture the intricate dynamics of living organisms with unprecedented clarity and minimal damage.
Methodology:
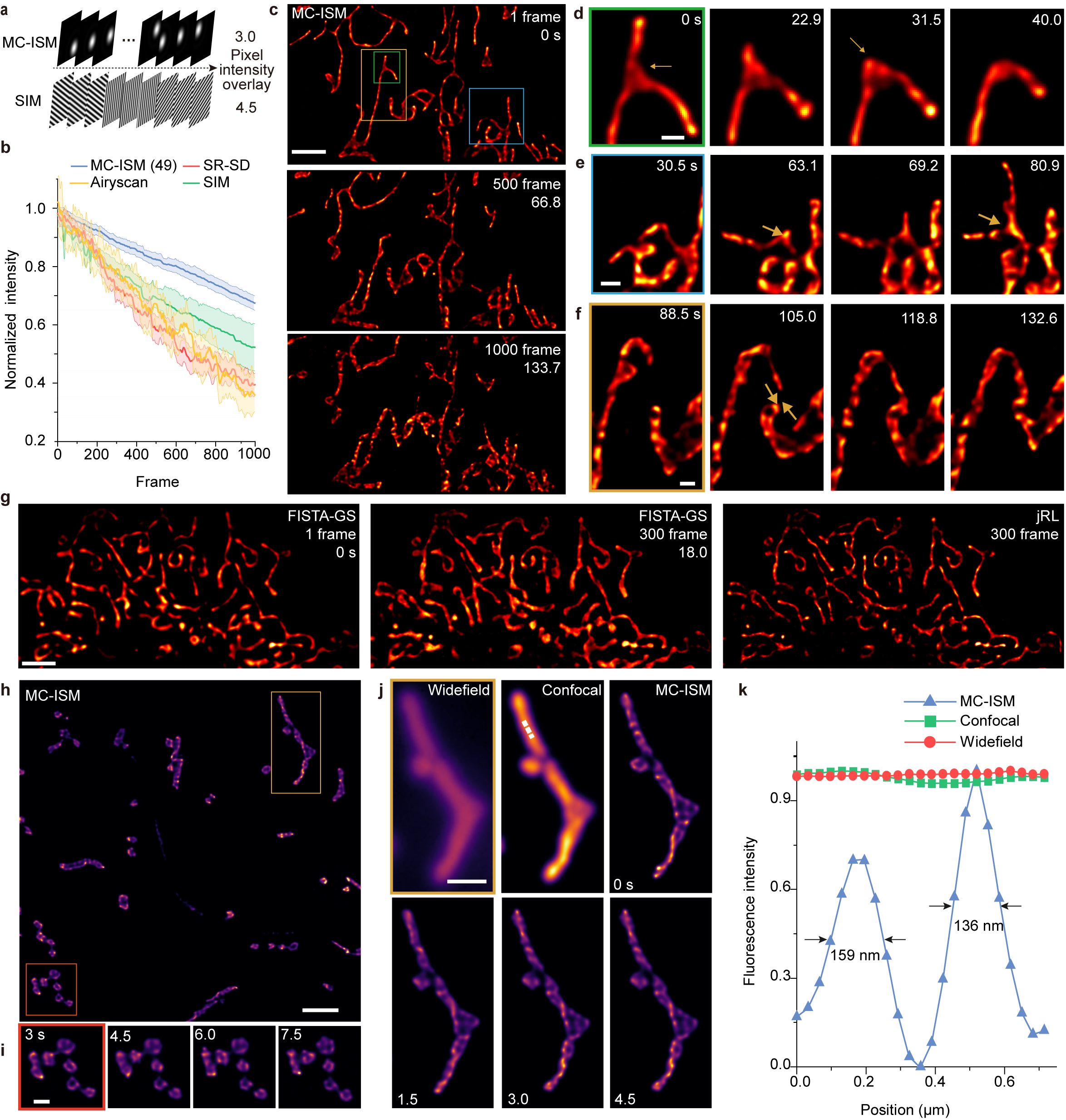
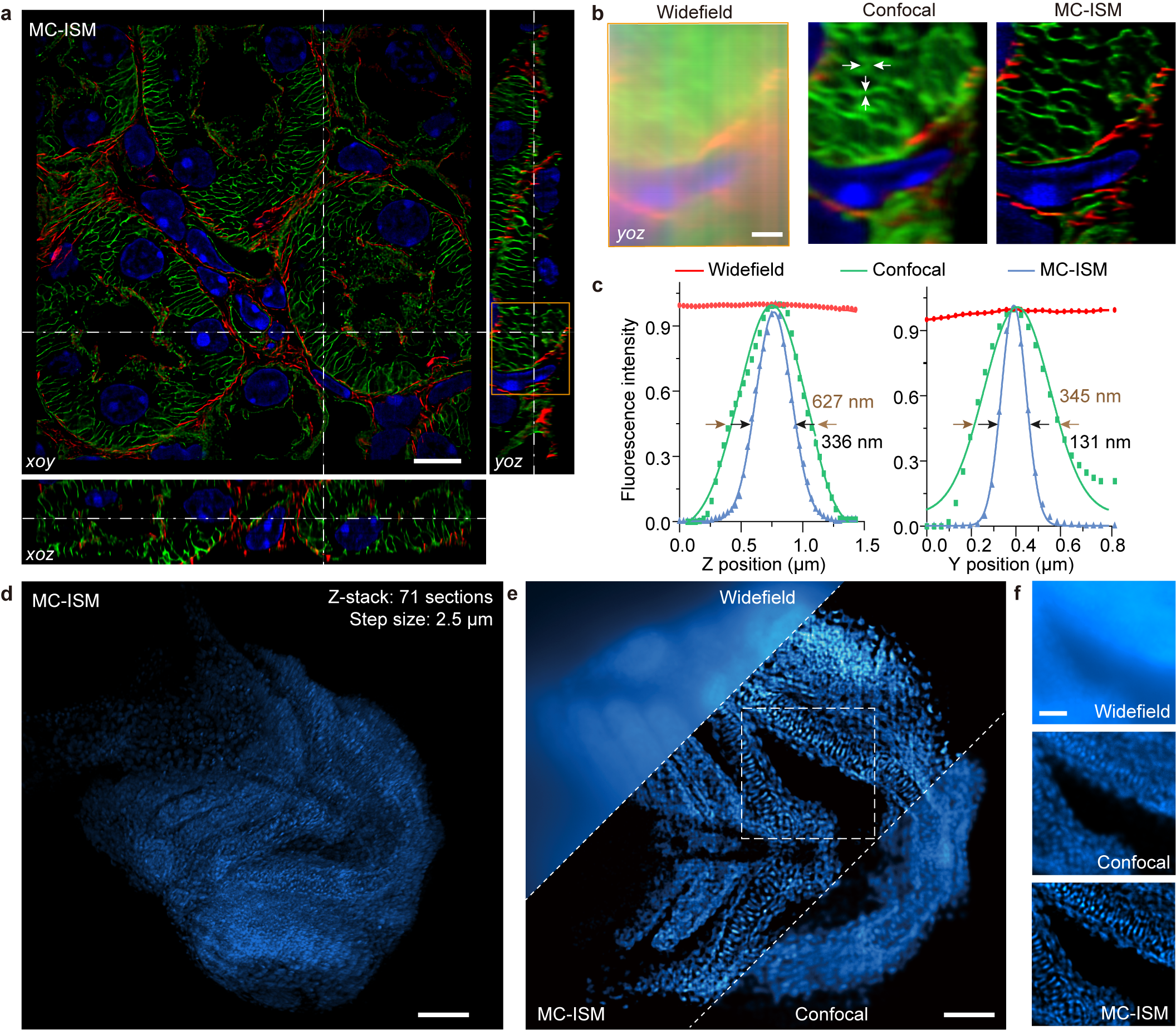
The research team utilized MC-ISM technology to perform three-color 3D super-resolution imaging on sections of mouse kidney. The technique operates within a volume of 66.5 μm × 66.5 μm × 12 μm, achieving an axial interval of 150 nm. By optimizing pinhole diameter and spacing, applying optical lock-in detection (OLID) to suppress out-of-focus signals, and utilizing a frame reduction reconstruction algorithm, MC-ISM significantly enhances imaging speed and quality. The imaging was also tested on DAPI-stained zebrafish heads and plant cells, showcasing its versatility and capability in deep tissue imaging.
Key findings:
MC-ISM improved lateral and axial resolutions to 131 nm and 336 nm, respectively, effectively suppressing out-of-focus signals and demonstrating optical sectioning comparable to spinning disk confocal microscopy.