Peking University, March 19, 2025: The research group led by Prof. Wang Chu from the College of Chemistry and Molecular Engineering at Peking University published a research article entitled “Quantitative Chemoproteomics Reveals Dopamine’s Protective Modification of Tau” in Nature Chemical Biology (DOI:10.1038/s41589-025-01849-9). Using a novel quantitative chemoproteomic strategy, the team uncovered a protective role of dopamine (DA) in regulating the function of the microtubule-associated protein Tau. This discovery deepens our understanding of dopamine’s physiological and pathological roles in the human brain.
Why it matters:
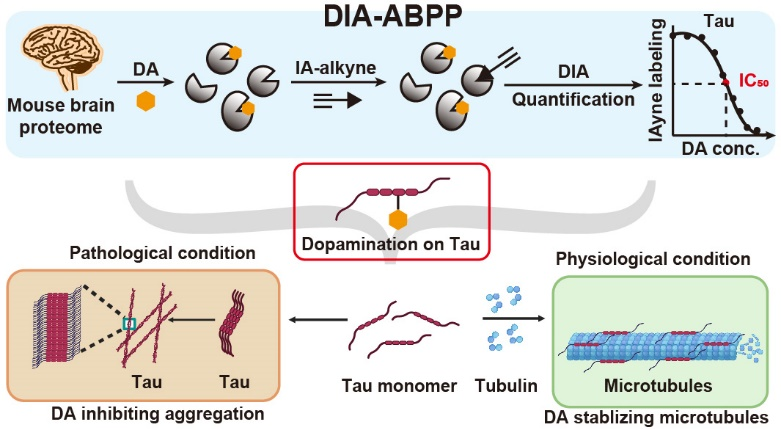
1. Dopamine, a critical neurotransmitter, is prone to oxidation, forming electrophilic products that react with proteins through a process termed dopamination.
2. While dopamine role in the nervous system is well-known, its functional impact on protein modifications, particularly Tau, has remained poorly understood.
The research:
1. The team developed a quantitative chemoproteomic strategy (DIA-ABPP) to globally measure dopamination at specific sites.
2. Using this approach, they identified and quantified over 6,000 dopamination sites, including two highly sensitive cysteines in Tau.
3. Biochemical validation revealed that dopamination of Tau prevents its amyloid fibrillation (a hallmark of neurodegenerative diseases) and promotes microtubule assembly, crucial for neuronal function.
4. Endogenous dopamination of Tau was also detected in mouse brains, confirming its physiological relevance.
Key findings and potentials:
1. Dopamine modifies Tau in a way that protects against harmful amyloid fibrillation and enhances microtubule assembly.
2. The study provides the first global portrait of dopamination, offering insights into dopamin’s physiological and pathological roles.
3. The findings have significantly contributed to the development of Tau-targeted therapies to treat neurodegenerative diseases such as Alzheimer’s.
*This article is featured in PKU News' "Why It Matters" series. More from this series.Click "here” to read the paper
Written by: Akaash Babar