Peking University, July 3, 2018: HIV is a virus that spreads through body fluids and attacks the body’s immune system. Without treatment, HIV infection progressively worsens over time, finally leading to AIDS.
Previous studies have found that for HIV-1 virus to form, a structural protein called Gag plays an important role. During HIV-1 assembly, Gag extensively clusters to form an immature protein coat at the plasma membrane. While evidence has shown that interactions between Gag and the viral RNA (vRNA) are crucial for assembly, the exact roles of these interactions have remained poorly understood.
Recently, using an advance superresolution imaging technique called Photoactivated Localization Microscopy (PALM), the laboratory led by Professor Antony K. Chen in the Department of Biomedical Engineering, College of Engineering, Peking University has deciphered the roles of Gag-vRNA interactions in HIV-1 virus assembly. Their research paper was published in PNAS on June 11, 2018.
“Because the diameter of a virus particle is only about 150 nanometers (nm), and the resolution of traditional microscopes is around 250 nm, the assembly mechanism of the virus cannot be clearly elucidated at the molecular level by conventional means,” said Prof. Chen, “So we employed PALM, an ultra-high resolution imaging technique that can illuminate a specific molecule with spatial resolution approaching 20 nm at the plasma membrane.”
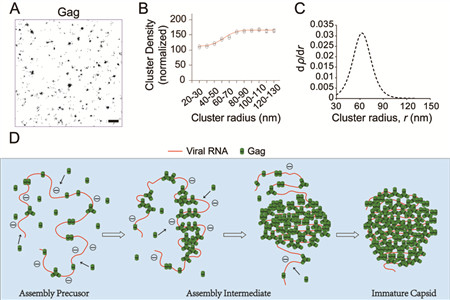
Caption of the image: PALM imaging and cluster formation analysis of the HIV-1 Gag protein. A) A representative PALM image of HIV-1 Gag. Individual spots represent single Gag molecules. (Scale bar = 500 nm) B) Gag cluster density increases asymptotically with increasing cluster radius. C) Analysis of transition in cluster growth of Gag. When the cluster radius reaches 63nm, the greatest change in cluster density is detected. D) Schematic model of how HIV-1 RNA scaffolds HIV-1 Gag assembly to form an immature capsid.
“We found that, in contrast to the general notion that vRNA only triggers initial Gag assembly and is dispensable for subsequent assembly, vRNA is indispensable throughout assembly, scaffolding the formation of assembly intermediates and maintaining their architectures via balancing of external forces acting on the assembly environment,” said Yang Yantao, a Ph.D. candidate in Professor Chen’s lab.
“This finding should advance the current understanding of HIV-1 and, potentially, other retroviruses,” said Professor Chen.
This project was supported by grants from the National Key R&D Program of China (2016YFA0501603 and 2016YFA0100702), the National Natural Science Foundation of China (31771583 and 81371613), and China’s 1000 Young Talent Award program.
Edited by: Zhang Jiang
Source: College of Engineering