Peking University, Oct 18, 2012: Peking University (PKU) Chemist Zhang Jun-Long and his co-workers have discovered the first example of weak intermolecular interactions (attractive π-π interaction) facilitated C-F Bond Activation. Their article “π-π Interaction Assisted Hydrodefluorination of Perfluoroarenes by Gold Hydride: A Case of Synergistic Effect on C—F Bond Activation” and its cover, were published on Journal of the American Chemical Society, (2012, 134, 16216-16227).
Synergistic effects between metal active sites or/and the surrounding amino acid residues includes hydrophobic effect, hydrogen bond, π-π Interaction and other weak interactions, which are prevalent in natural metalloenzymes activating small unreactive molecules. Learning such approaches inspires us to enrich the realm of coordination chemistry and catalysis and promotes the development of organo/metal binary catalytic systems to unprecedented organic transformations.
Activation of C-F bond by transition metals attracts much attention, providing the potential new routes to fluorinated organics, which are important in pharmaceutics and material sciences. However, for the strong bond strengths, activation of C-F bonds still remains challenges. Late transition metals (group 8-10) may be favor to perform catalytically functionalizing C-F bonds for their M-F bonds are not prohibitively strong. To expand the scope of late transition metals, Zhang Jun-Long’s group firstly reported group 11 metal complexes, gold, to catalyze hydrodefluorination (HDF) of perfluoroarenes efficiently (TON is up to 1000, Adv. Synth. Catal. 2012, 354, 1529—1541).

Figure 1
To further understand the role of gold hydride in C-F bond activation, they investigated the reactivity of gold hydrides [(NHC)AuH] (NHC= N-heterocyclic carbine) as model complexes toward the HDF of perfluoroarenes. To their surprise, the unual π-π interaction assisted C-F bond activation introduced by DMAP is effective to coorperate with [(IMes)AuH] in HDF of perfluoroarenes. Through UV-vis spectral titrations and 1H-NMR spectroscopic studies, the possible charge transfer complexes between DMAP and perfluoroarenes were suggested to be crucial to promote hydrogen transfer from [(NHC)AuH] to pyridyl N atom, associated with C—F bond cleavage. The interpretation of π-π interaction assisted C-F activation is supported by the reduced activation barriers computed with three component “[(NHC)AuH] + DMAP + perfluoroarenes” through DFT calculation. They proposed a new pathway representing a further mechanistic possibility for the activation of C-F bonds by transition metals. (J. Am. Chem. Soc. 2012, 134, 16216-16227).
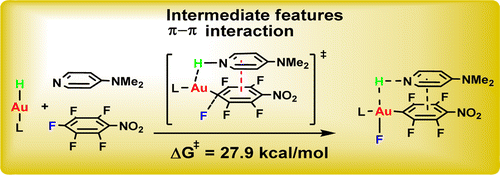
Figure 2.
This project was supported by National Key Basic Research Support Foundation of China (NKBRSFC) (2010CB912302) and the National Scientific Foundation of China (grant no.20971007).
Edited by: Zhang Jiang
Source: College of Chemistry and Molecular Engineering