Peking University, Sep 3, 2012: Led by Professor Enge Wang, a research team at PKU lately reported their new progress on the adsorption properties of ice surface, which was published in Proceedings of the National Academy of Sciences (PNAS 109, 201206879 (2012), http://www.pnas.org/cgi/doi/10.1073/pnas.1206879109).
Ice is one of the most abundant and well-known materials on earth. It plays a critical role in many of the physical and chemical processes throughout the universe, such as interstellar phenomenon, life in cryosphere and regulation in global climate, thus has attracted extensive attentions. Despite progress in understanding the mechanism and formation of ice over the years, our knowledge of the structure and physical/chemical properties of ice surface is still limited.
In the past few years, Prof. Enge Wang’s group has made significant progress in the study of ice, including a new characterization of ice surface (Phys. Rev. Lett. 101, 155703 (2008)) and an observation of amorphous phenomenon of ice surface (NATURE Materials 10, 794 (2011)), which is of curial importance for the understanding the proton order-disorder transition and the pre-melting process of ice surface.
Recently, ICQM faculty member Enge Wang and Limei Xu together with their students Zhaoru Sun and Ding Pan made new progress on the adsorption properties of ice surface. They showed that adsorption on ice surface is affected not only by local dangling atoms on ice surface, but also by dangling atoms on the entire surface. This is due to the fact that the interaction between the surface and admolecule caused by dangling atoms is long ranged, thus consideration of only the nearest dangling atoms (as in previous studies) is not enough to describe the adsorption property of ice surface correctly. They further found a positive correlation between adsorption energy and heterogeneity of the ice surface, which suggests that admolecules are more likely to be adsorbed on ice surface with larger heterogeneity. This work helps to reveal the possible mechanism of ice growth on molecular scale, and also helps to explain the adsorption mechanism of polar molecules in clouds associated with global climate.
Since its publication, the paper has attracted considerable attentions. Prof. Enge Wang is invited to give talk on ice in the ACS meeting 2012 (Philadelphia) and Conference on Computational Physics 2012 (CCP2012, Kobe).
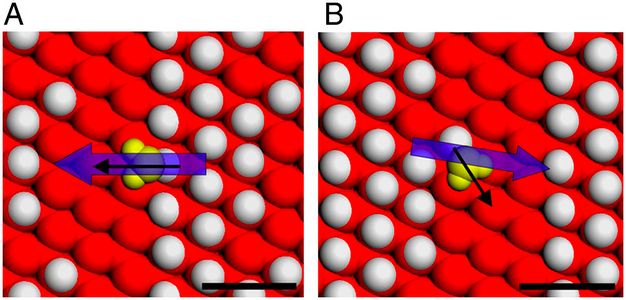
Relative orientation of the dipole moment of the adsorbed water molecule and the local electric field at different adsorption sites on the same surface. (A) Stronger enhancement of adsorption due to a lineup of local electric field with the dipole moment, and (B) a weaker enhancement of adsorption of water molecule.
Edited by: Zhang Jiang
Source: School of Physics