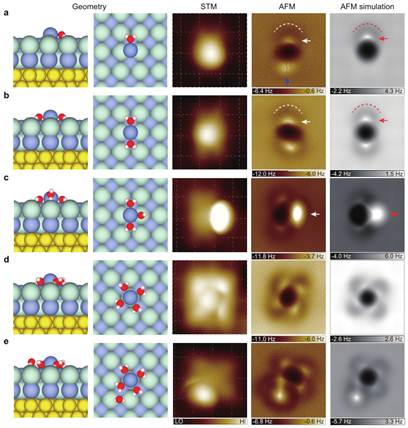
Figure 2 The atomically resolved images of different Na+ ion hydrates. From the left to right column: atomic model (Geometry), scanning tunneling microscopy (STM), atomic force microscopy (AFM) and AFM simulation. Image size: 1.5 nm ×1.5 nm.
Furthermore, the researchers compared the mobility of different ion hydrates in a well-controlled manner via the inelastic electron tunneling technique. They found an interesting magic-number effect: the Na+ hydrated with three water molecules diffuses one to two orders of magnitude faster than other Na+ hydrates and even much faster than the Na+ in dilute bulk solution (Figure 3). Ab initio calculations and MD simulations revealed that such high ion mobility arises from the degree of the symmetry match between the hydrate and substrate. The magic-number effect applies within a wide range of temperatures (up to room temperature) according to the classical MD simulations. Besides, they found that the magic-number effect applies for many salt ions, suggesting its generality.
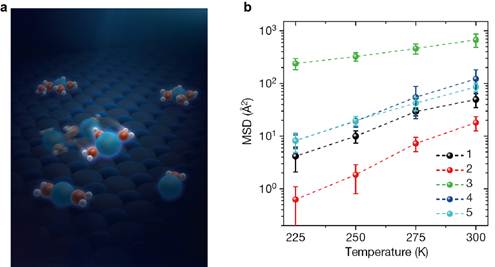
Figure 3 Magic number effect in the transport of ion hydrates. a, Schematic showing that the Na+ hydrated with three water molecules diffuses much faster than other Na+ hydrates. b, Mean Square Displacement (MSD) in 1 ns of Na+•nH2O (n = 1–5) between 225 K and 300 K.
This work established, for the first time, direct correlation between the atomic structure and transport mechanism of hydrated ions, which may completely renovate the traditional understanding of ion transport in nanofluidic systems. In addition, those results point out a new way to control the ion transport in nanofluidic systems by interfacial symmetry engineering, which is of great importance for an extremely wide range of technologically and biologically relevant processes, including corrosion, water desalination, electrochemistry, and biological ion channel, etc.. The techniques developed in this work can be easily extended to different ions and other hydration systems, opening up the possibility of studying various hydration processes down to atomic scale.
Dr. Peng Jinbo (now a Humboldt fellow), Cao Duanyun of International Center for Quantum Materials (ICQM) of Peking University and He Zhili of College of Chemistry and Molecular Engineering of Peking University are the co-first authors of this work. Professor Jiang Ying (SPM), Professor Wang Enge (DFT), Professor Gao Yiqin (MD) and Professor Xu Limei (DFT) are the co-corresponding authors. This work received supports from National Natural Science Foundation of China, Ministry of Science and Technology of China, Chinese Academy of Sciences, and Collaborative Innovation Center of Quantum Matter.
Paper link: J. Peng, D. Cao, Z. He, J. Guo, P. Hapala, R. Ma, B. Cheng, J. Chen, W.-J. Xie, X.-Z. Li, P. Jelínek, L.-M. Xu*, Y.-Q. Gao*, E.-G Wang*, Y. Jiang*, "The effect of hydration number on the interfacial transport of sodium ions", Nature, DOI: 10.1038/s41586-018-0122-2 (2018) (https://doi.org/10.1038/s41586-018-0122-2)
Edited by: Zhang Jiang
Source: School of Physics